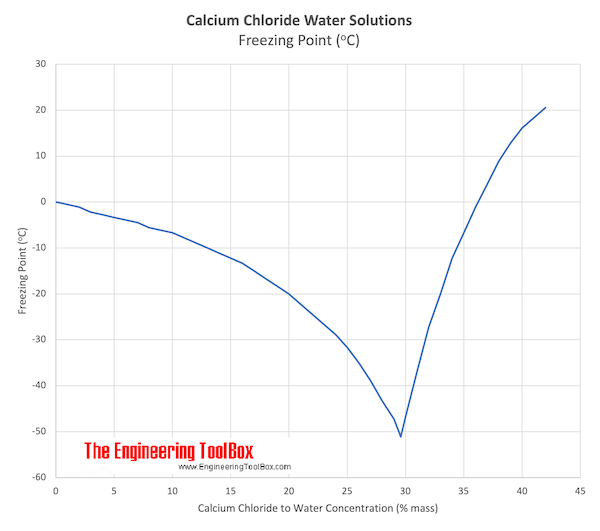
Freezing point of milk: Freezing point of a solution depends on the number of particles in the solvent (water phase of milk), rather than the kind of particles.


What is the freezing point of water or melting point of water? Are the freezing and melting point the same? Here’s the answer to these questions.
The density of ocean water is determined by its salinity (or salt content) and temperature. The saltier and/or colder the water is, the denser it is. Salt water is most dense at its freezing point, unlike fresh water, which is most dense at about 3.9°C (39.0

/IceCubes-58dd5a443df78c51620bcf7c.jpg)
Many living organisms are able to tolerate prolonged periods of time at temperatures below the freezing point of water. Most living organisms accumulate cryoprotectants such as anti-nucleating proteins, polyols, and glucose to protect themselves against frost damage by sharp ice crystals.
Freezing-point depression is the decrease of the freezing point of a solvent on addition of a non-volatile solute.Examples include salt in water, alcohol in water, or the mixing of two solids such as impurities into a finely powdered drug.


reer Aociation www reerAociationorg ® For more information on draught system cleaning or other components of a draught beer system, visit the rewers ssociations Draught eer uality anual at: www.draughtuality.org
We throw salt on the ground to prevent ice from forming. Ice does not form because salt lowers the freezing temperature of water. But how does it do this?
Freezing point: Freezing point,, temperature at which a liquid becomes a solid. As with the melting point, increased pressure usually raises the freezing point. The freezing point is lower than the melting point in the case of mixtures and for certain organic compounds such as fats.

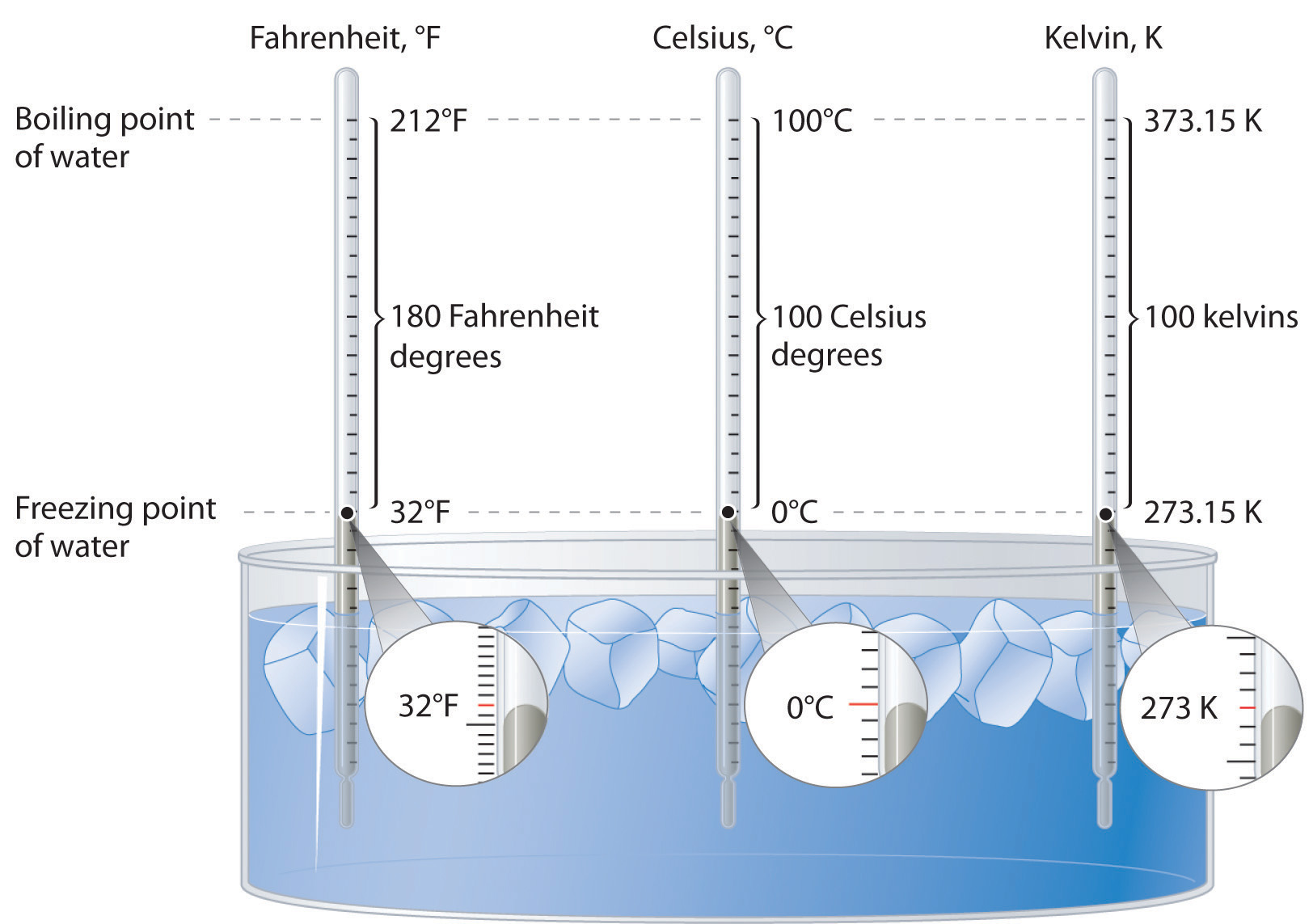
The normal boiling point of water is 100 o C because this is the temperature at which the vapor pressure of water is 760 mmHg, or 1 atm. Under normal conditions, when the pressure of the atmosphere is approximately 760 mmHg, water boils at 100 o C.
Seen and Heard. What made you want to look up freezing point?Please tell us where you read or heard it (including the quote, if possible).